This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
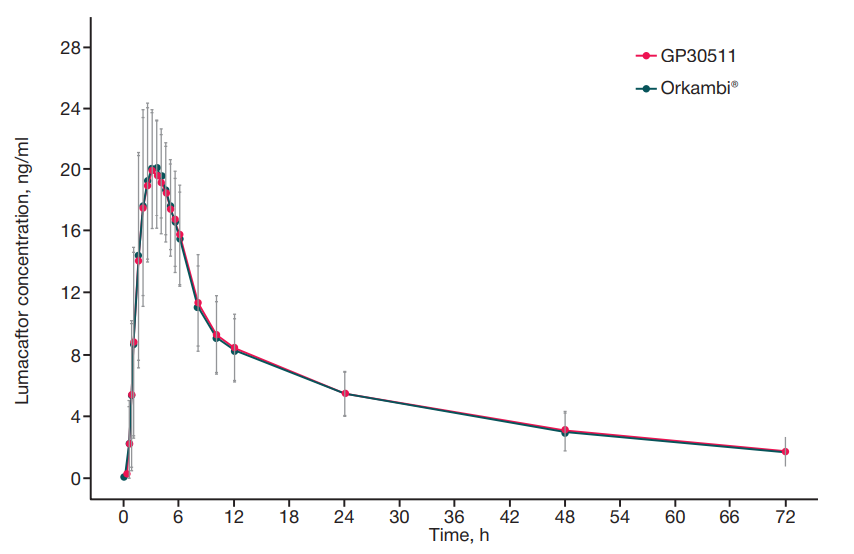
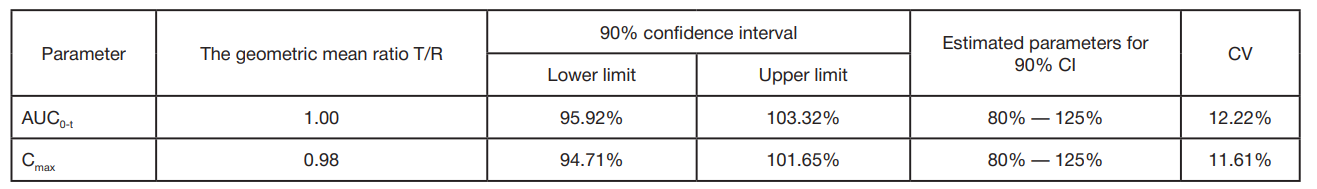
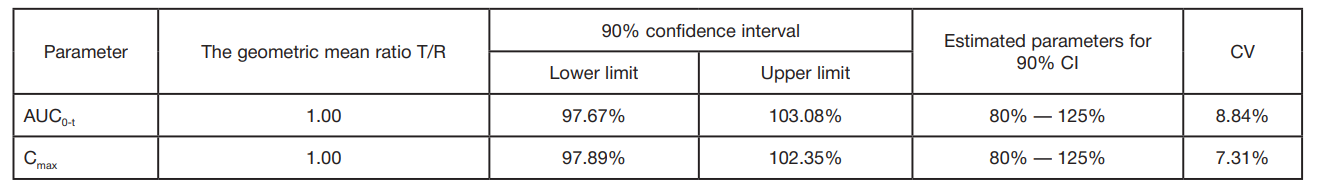
A reproduced combination of ivacaftor and lumacaftor, CFTR protein modulators. Ethical and pharmacokinetic aspects
1 Clinical Hospital No. 3, Yaroslavl, Russia
2 Pharm Holding CJSC, St. Petersburg (Pharm Holding CJSC), Russia
Correspondence should be addressed: Kseniia S Radaeva
Svyasi Str., 34a, Strelna village, Saint-Petersburg, 198515, Russia; moc.mrahporeg@aveadaR.aiinesK
Funding: the study was funded by LLC “GEROPHARM”.
Author contribution: Arefeva AN and Radaeva KS conceived of the presented article. Arefeva AN conceived and planned the trial. Radaeva KS wrote the manuscript with input from all authors. Arefeva AN and Noskov SM collected and processed data. Radaeva KS conducted a comprehensive review of the existing literature on the topic. Arefeva AN analysed data. All authors edited the paper and contributed to the final manuscript.
Compliance with ethical standards: the condition for conducting the clinical trial was authorization from the Ministry of Health of the Russian Federation No. 212 dated 04/17/2023 and approval of the study by Independent Ethics Committee (excert from the meeting protocol of the Ethics Committee No. 325 dated 01/17/2023). All the essential trial documents (protocol GP30511-P4–01–01, Investigator’s Brochure, written information given to trial subjects and informed consent form, volunteer life and health insurance certificate) were provided and approved by the Independent Ethics Committee (IEC) of the research center according to the procedures of this committee. The researchers are obliged not to disclose personal and medical data of the subjects. Prior to the start of any trial procedures, an informed consent procedure was carried out in accordance with the principles of the Declaration of Helsinki, ICH recommendations and national regulatory standards. Each volunteer included in the study was insured and must have received an original insurance certificate.