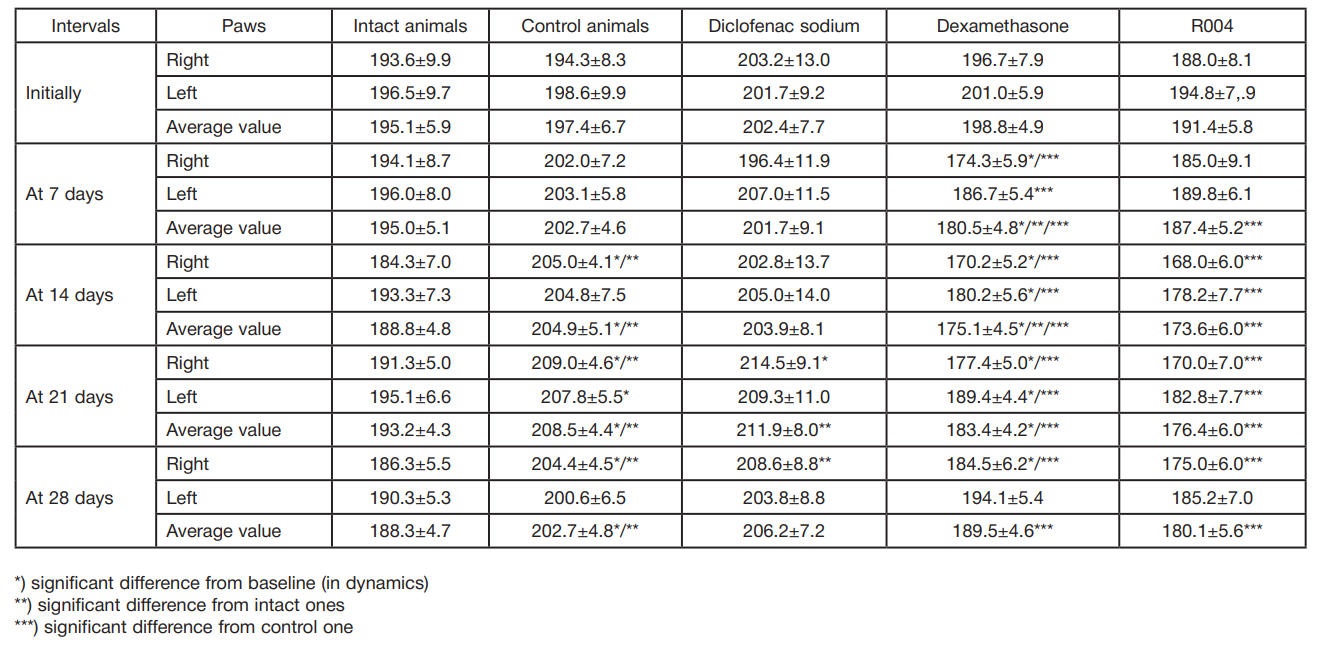
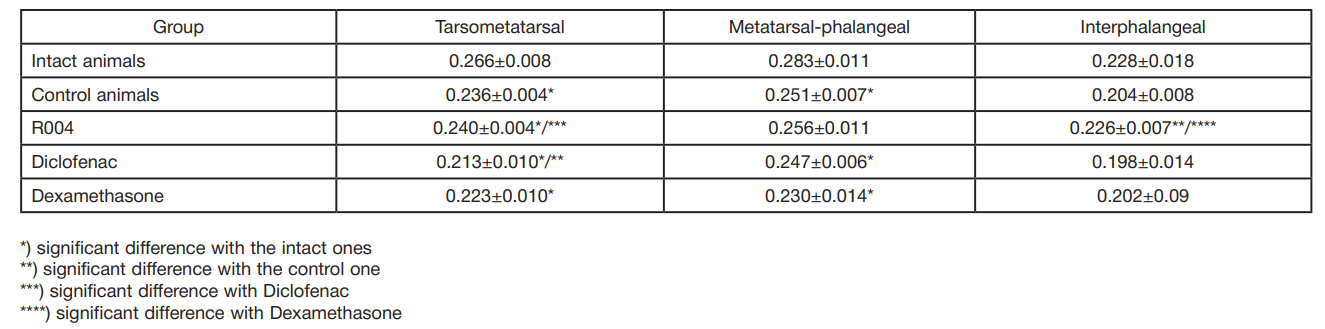
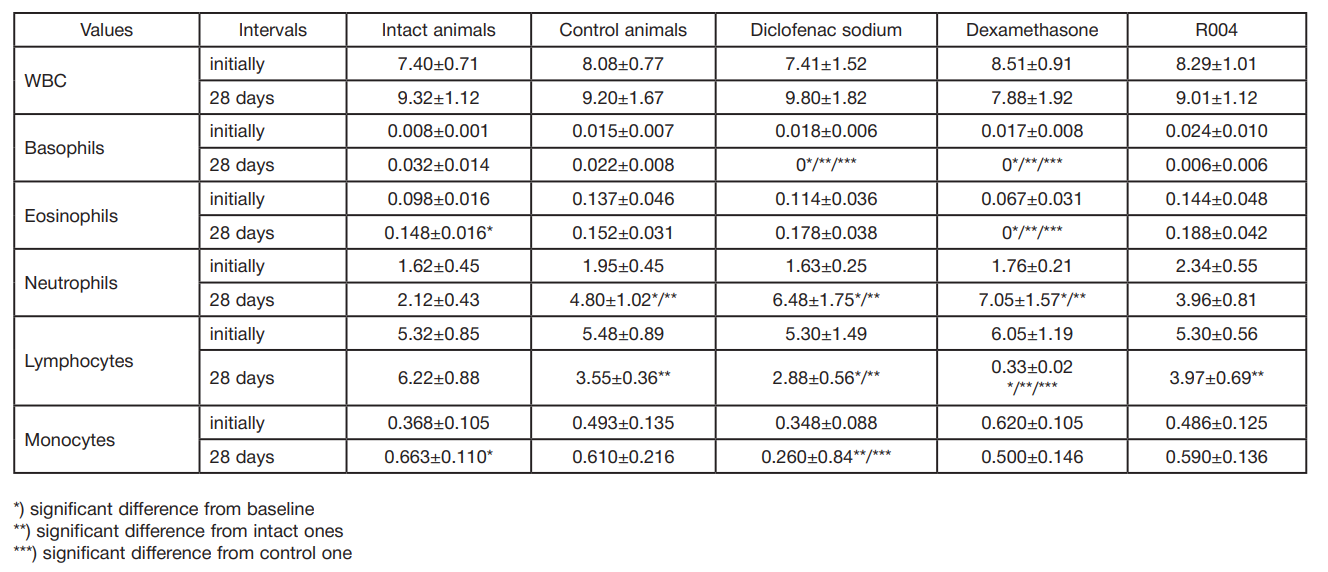
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
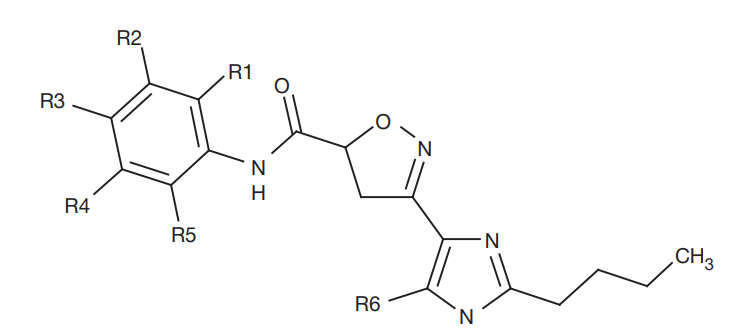
Antirheumatic activity of 3-imidazole-substituted-4,5-diaryloisoxazol-3-carboxylic acid amide derivative, a proteinase inhibitor-activated type II receptor
1 Yaroslavl State Pedagogical University named after KD Ushinsky
2 Yaroslavl State Medical University, Yaroslavl, Russia
3 Grodno State Medical University, Grodno, Belarus
4 Russian University of Medicine, Moscow, Russia
Correspondence should be addressed: Nikita N Volkhin
Technopark str., 11/2, Yaroslavl, 150030, Russia; ur.umsy@nihklovnn
Funding: this study was supported by the Ministry of Health of the Russian Federation (state assignment 1022051600008-9-3.1.5;3.2.22) “Development of a drug for the treatment of rheumatoid arthritis and other inflammatory diseases”.
Acknowledgement: the work was carried out in collaboration with the Scientific Department of the Institute of Pharmacy of Yaroslavl State Medical University, Yaroslavl.
Author contribution: Fedorov VN — idea development, analysis and layout of the collected material; Korsakov MK — writing the text; Shetnev AA — a general idea and planning of the article, synthesis of the compound under study; Grechishcheva OV — conducting an experiment; Vdovichenko VP — collection and primary analysis of material, working with literature; Volkhin NN — conducting an experiment; Smirnov NA — primary analysis of the collected material; Khokhlova AA — collection of primary material, design of the list of references; Arshinov AV — text editing.
Compliance with ethical standards: the study was carried out in compliance with all ethical standards recommended in the Russian Federation. Rats were selected as a test system, as animals with a minimum set of characteristics that make it possible to conduct an experiment: a sufficient size of paws for convenient measurements and possibility of taking the volume of blood necessary for the study. The animals were kept in cages of sufficient area and with timely bedding change (2 times a week). Animals are provided with free access to water and food, a 12-hour lighting cycle, optimal temperature and humidity, and supervision by a licensed veterinarian. Although the research protocol did not allow to use painkillers that could distort the results of experiments, all procedures were carried out by qualified and experienced personnel, which ensured minimization of stress and pain. The animal study was preceded by in vitro studies of the drug. The power of the statistical tests used was evaluated, which made it possible to form samples of an optimal size. The animal study was approved by the Independent Ethical Committee of the Federal State Budgetary Educational Institution of Higher Education Yaroslavl State Medical University of the Ministry of Health of the Russian Federation, Protocol No. 6 dated 09/14/2023.