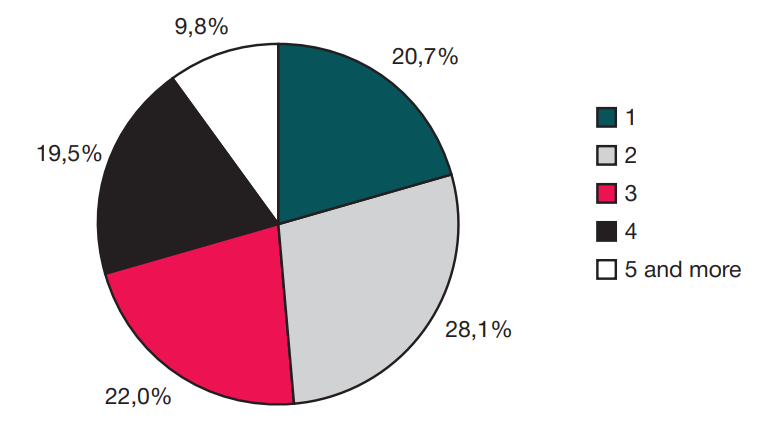
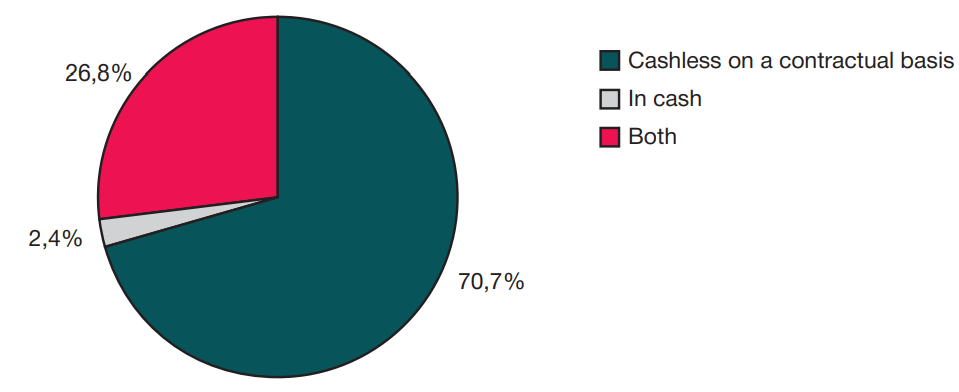
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
REVIEW
Early phase clinical research as viewed by healthy volunteers
Рeoples’ Friendship University of Russia (RUDN University), Moscow, Russia
Correspondence should be addressed: Dmitry A. Kliuev
ul. Miklukho-Maclay, 6, Moscow, 117198, Russia; moc.liamg@694070veulkjirtimd
Author contribution: Fitilev SB — development of article concept, analysis of scientific data, article editing; Vozzhaev AV — development of article concept, analysis of scientific data, writing an article; Shkrebniova II — collection of data, analysis of scientific data, writing an article; Kliuev DA — review of the article-related publications, statistical calculations, writing an article; Saakova LN — collection of data, review of the article-related publications.
Compliance with ethical standards: voluntary informed consent was obtained from every participant. Questioning was conducted on a voluntary basis.