This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
REVIEW
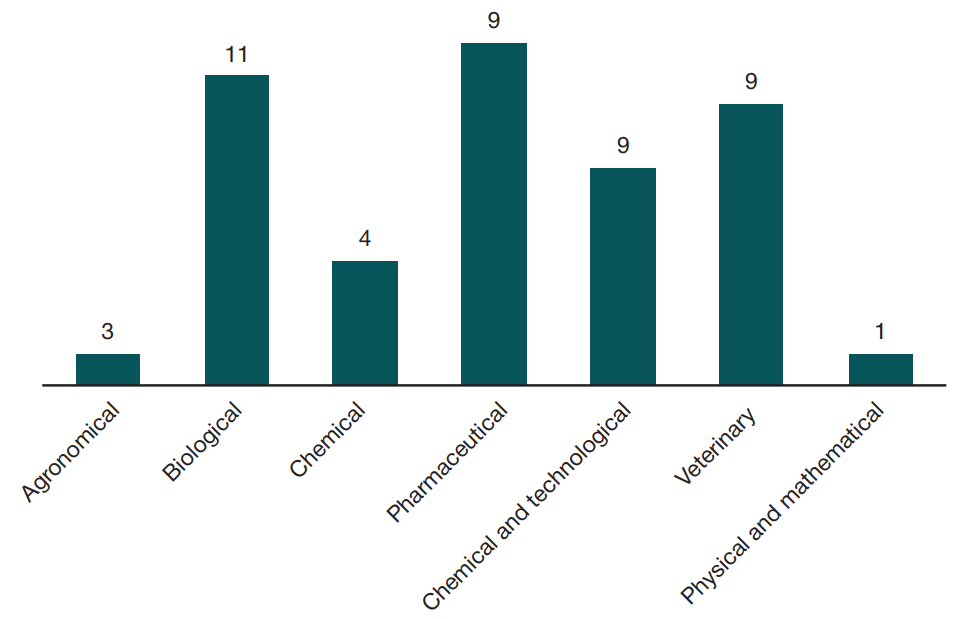
Developing the system of state control of drug quality through formation of competence centers in federal laboratories of the Information Center for Expertise, Accounting and Analysis of Circulation of Human Medicinal Products of the Federal Supervisory Agency for Healthcare of Russia
1 Information Center for Expertise, Accounting and Analysis of Circulation of Human Medicinal Products of the Federal Supervisory Agency for Healthcare, Moscow, Russia
2 Yaroslavl State Medical University, Yaroslavl, Russia
Correspondence should be addressed: Elena G. Lileeva
ul. Popova, 24, Yaroslavl, 150010, Russia; ur.xednay@6002aveelile
Author contribution: Galeev RR — article concept, study planning; Lileeva EG — literature selection and analysis; Ryzhkova EA — data generalization, text writing; Galeeva EV — data collection, data analysis; Lezhnina NA — data interpretation, preparing a draft of the manuscript.